Room temperature storage statements for products with a storage statement reading store at controlled room temperature the labeling should read as follows on the package insert.
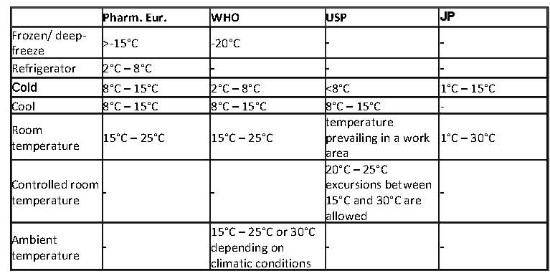
Controlled room temperature european pharmacopoeia.
The general notices apply to all monographs and other texts of the european pharmacopoeia.
The european pharmacopoeia pharm eur.
Ulrich c richard poska c and arminda montero c abstract.
The european pharmacopoeia defines it as being simply 15 to 25 c 59 to 77 f and the japanese pharmacopeia defines ordinary temperature as 15 to 25 c 59 to 77 f with room temperature being 1 to 30 c 34 to 86 f.
Excursions between 15 and 30 59 and 86 f that are experienced in pharmacies hospitals and warehouses and during shipping are.
Usp controlled room temperature range expansion desmond g hunt a chris chandler b david a.
Control of impurities in substances for pharmaceutical use.
The temperature maintained thermostatically that encompasses at the usual and customary working environment of 20 25 68 77 f.
Ich guidance for industry q1a r2.
According to european compliance academy a temperature excursion is the deviation from the labelled storage condition of a product for any duration whether during transportation or distribution.
The temperature maintained thermostatically that encompasses at the usual and customary working environment of 20 25 68 77 f.
The temperature maintained thermostatically that encompasses at the usual and customary working environment of 20 25 68 77 f.
Store at 20 c to 25 c 68 f to 77 f excursions permitted between 15 c and 30 c between 59 f and 86 f.
This article provides a brief overview of drug product stability studies and practices with a focus on temperature control during storage and distribution.
Excursions between 15 and 30 59 and 86 f that are experienced in pharmacies hospitals and.
Yet in developing countries where temperature and humidity can be far outside this range there is.
Health canada the european free trade area efta and the world health organization 2 in this application note we look at the fda regulations and ich guidelines that address supply chain management for temperature controlled pharmaceutical and biotechnical products including.
Calculated not to be more than 25 c and that allows excursions between 15 and 30 c.
The european pharmacopoeia pharm eur gives some hints in chapter 1 2.
And other ich sponsors.
United states pharmacopoeia.